Over 70 million people in Europe experience tinnitus, for 7 million it creates a debilitating condition. Severe tinnitus is often associated with depression, anxiety and insomnia, resulting in an enormous socio-economic impact (Langguth, 2011). It has been estimated that 13 million people in Western Europe and in the US approach their doctor regularly because of their tinnitus symptoms (Vio and Holme, 2005). These figures are expected to increase due to increasing occupational and leisure noise and due to the demographic development.
In spite of its enormous socioeconomic relevance funding for tinnitus research has been very limited (Cederroth et al., 2013). Now the European Union has approved funding for the COST Action TINNET, which aims to create a paneuropean tinnitus research network. The COST (Cooperation in Science and Technology) program has been founded in 1972 and is Europe's longest-running research program. COST is an intergovernmental framework for European Cooperation in Science and Technology, which promotes and coordinates nationally funded research in Europe through funding of research networks. One of the key scientific areas covered is Biomedicine and Molecular Biosciences (BMBS). The acronym TINNET stands for the planned Tinnitus research Network, but also for the goal of the action, namely New Treatments for Tinnitus.
Why TINNET now?
 Recent advances in neuroscientific methods have led to substantial progress in the understanding of tinnitus. Both animal models of tinnitus and neuroimaging research in patients suffering from tinnitus have provided important insight into the neuronal mechanisms involved in the pathophysiology of tinnitus. In short, alterations of neural along the central auditory pathways have been observed in connection with tinnitus (Eggermont and Roberts, 2004). These changes of neural activity seem to arise from dysfunctional activation of neural plasticity induced by altered sensory input, namely auditory deprivation in most cases (Norena, 2011), but also altered somatosensory input (Shore et al., 2007) in some patients. The mentioned adaptive processes are mainly driven by mechanisms of homeostatic plasticity which alter the balance between excitatory and inhibitory function of the auditory system at several levels in order to compensate for the reduced input (Norena, 2011).
Recent advances in neuroscientific methods have led to substantial progress in the understanding of tinnitus. Both animal models of tinnitus and neuroimaging research in patients suffering from tinnitus have provided important insight into the neuronal mechanisms involved in the pathophysiology of tinnitus. In short, alterations of neural along the central auditory pathways have been observed in connection with tinnitus (Eggermont and Roberts, 2004). These changes of neural activity seem to arise from dysfunctional activation of neural plasticity induced by altered sensory input, namely auditory deprivation in most cases (Norena, 2011), but also altered somatosensory input (Shore et al., 2007) in some patients. The mentioned adaptive processes are mainly driven by mechanisms of homeostatic plasticity which alter the balance between excitatory and inhibitory function of the auditory system at several levels in order to compensate for the reduced input (Norena, 2011).
In addition to altered activity in central auditory pathways, recent research has increasingly identified further central nervous system components of tinnitus (Langguth et al., 2013). Brain networks involved in tinnitus pathophysiology differ from patient to patient and include the frontal cortex, the anterior cingulate, the insula and the medial temporal lobe including amygdala, hippocampus and parahippocampus (De Ridder et al., 2013). These brain areas are related to attention-, salience-, distress- and memory-related aspects of tinnitus. Depending on the individual clinical characteristics different networks seem to be involved to a different extent (De Ridder et al., 2013).
 This increasing knowledge prompted the development of innovative treatment approaches in the last years. All these methods have in common that they aim at targeted modulation of the neuroplastic changes underlying tinnitus, either by specific auditory stimulation, by conditioned learning, by pharmacologic treatment, by brain stimulation or by a combination of different treatments. Promising results in pilot studies illustrate the potential of the various approaches. However, most results are characterized by a high interindividual variability, indicating the relevance of tinnitus subtypes which differ in their response to specific treatments. Thus there is an urgent need for identifying pathophysiologically distinct subgroups for optimizing and further validating these treatment interventions. Moreover the identification of valid subtypes will enable to better predict treatment response to the various currently already available treatment. Thus further progress is hampered by the fact that tinnitus represents a highly heterogeneous condition. Thus many findings coming from small samples cannot be replicated and remain inconclusive. This problem is related to the current research structure with many separate groups working largely in isolation and being funded locally or nationally. In order to effectively address the heterogeneity of tinnitus a coordinated effort is required to enable the analysis of large samples. Within the TINNET project networking and capacity building activities will be supported, in order to transform the currently still separated research groups into an efficient research network.
This increasing knowledge prompted the development of innovative treatment approaches in the last years. All these methods have in common that they aim at targeted modulation of the neuroplastic changes underlying tinnitus, either by specific auditory stimulation, by conditioned learning, by pharmacologic treatment, by brain stimulation or by a combination of different treatments. Promising results in pilot studies illustrate the potential of the various approaches. However, most results are characterized by a high interindividual variability, indicating the relevance of tinnitus subtypes which differ in their response to specific treatments. Thus there is an urgent need for identifying pathophysiologically distinct subgroups for optimizing and further validating these treatment interventions. Moreover the identification of valid subtypes will enable to better predict treatment response to the various currently already available treatment. Thus further progress is hampered by the fact that tinnitus represents a highly heterogeneous condition. Thus many findings coming from small samples cannot be replicated and remain inconclusive. This problem is related to the current research structure with many separate groups working largely in isolation and being funded locally or nationally. In order to effectively address the heterogeneity of tinnitus a coordinated effort is required to enable the analysis of large samples. Within the TINNET project networking and capacity building activities will be supported, in order to transform the currently still separated research groups into an efficient research network.
How will TINNET work?
The aim of TINNET is the identification of pathophysiologically and clinically meaningful subtypes of tinnitus. This in turn is required for increasing the efficacy of currently available treatments by assigning the most effective treatment to the individual patient.
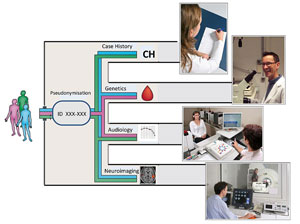
The strategy of TINNET is to standardize and coordinate clinical, neuroimaging and genetic assessment of tinnitus patients and to aggregate data in a large scale database in order to identify tinnitus subtypes and their neurobiological underpinnings. This will facilitate the developments of new therapies and improve the efficacy of currently available treatments.
Five different workgroups will be established which will focus on the following objectives
- Clinical and audiological assessment of tinnitus patients according to common standards
- Data management in a central database and identification of subtype candidates
- Developing standards for neuroimaging studies and probing the neurobiological entity of the defined subtypes by large-scale analyses of neuroimaging data
- Identifying the involvement of genetic factors in the pathogenesis of the different subtypes of tinnitus
- Development of standards for outcome measurements in clinical trials and central data collection
The expected result is the identification of meaningful subtypes of tinnitus and their neurobiological underpinnings. This in turn will improve tinnitus treatment by enabling to better assign current and to better tailor future treatments to specific patient subgroups. Since the high heterogeneity of tinnitus is considered as the major obstacle for the development of more effective treatment strategies, TINNET will address the current bottleneck in the development process of innovative treatment approaches. This requires a coordinated collaborative effort from clinicians and technicians of different centers and disciplines and bio- and health-tech companies working together in ongoing close collaboration.
What are the next steps?
TINNET started with the constitution of the management committee in April 2014. We hope that the program will attract many clinicians and researchers and will motivate them to join forces in order to work for a better therapy for the many people worldwide who suffer from tinnitus.
References
Cederroth, C.R., Canlon, B., Langguth, B., 2013. Hearing loss and tinnitus-are funders and industry listening? Nat Biotechnol 31(11), 972-974.
De Ridder, D., Vanneste, S., Weisz, N., Londero, A., Schlee, W., Elgoyhen, A.B., Langguth, B., 2013. An integrative model of auditory phantom perception: Tinnitus as a unified percept of interacting separable subnetworks. Neurosci Biobehav Rev.
Eggermont, J.J., Roberts, L.E., 2004. The neuroscience of tinnitus. Trends Neurosci. 27(11), 676-682.
Langguth, B., 2011. A review of tinnitus symptoms beyond 'ringing in the ears': a call to action. Curr.Med.Res.Opin. 27(8), 1635-1643.
Langguth, B., Kreuzer, P.M., Kleinjung, T., De Ridder, D., 2013. Tinnitus: causes and clinical management. Lancet Neurol 12(9), 920-930.
Norena, A.J., 2011. An integrative model of tinnitus based on a central gain controlling neural sensitivity. Neurosci.Biobehav.Rev. 35(5), 1089-1109.
Shore, S., Zhou, J., Koehler, S., 2007. Neural mechanisms underlying somatic tinnitus. Prog.Brain Res. 166, 107-123.
Vio, M.M., Holme, R.H., 2005. Hearing loss and tinnitus: 250 million people and a US$10 billion potential market. Drug Discov.Today 10(19), 1263-1265.